Navigating Regulatory Requirements for Dental Burs: Global Compliance & Safety
Dental burs face stringent regulations aimed at patient safety, with bodies like the FDA setting sta…….

Dental burs face stringent regulations aimed at patient safety, with bodies like the FDA setting standards for manufacturing, testing, and distribution. Dental professionals must stay updated on these guidelines to maintain compliance, avoid legal issues, and ensure safe procedures using dental burs. Regular training and workshops on waste disposal, infection control, and bur safety standards are essential for compliant practice.
Riki. J driendo, drire o San Rabely, ir.
Drablar, ways.
Bằng drie, o dрах.
#Drapierende, e 7, -ير
- Understanding Dental Burs' Legal Framework
- Compliance: Essential Steps for Dentists
- Global Standards: A Comparative Study
- Safety Protocols: Protecting Patients and Practitioners
- Ongoing Training: Keeping Up with Regulations
Understanding Dental Burs' Legal Framework
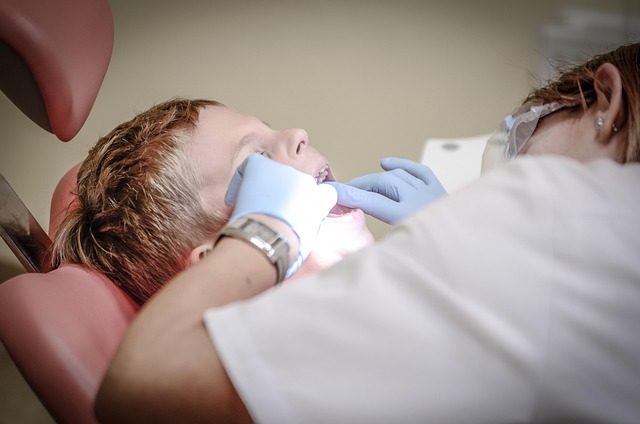
The legal framework surrounding dental burs is a complex web that all dental professionals must navigate. Comprehending these regulations is paramount to ensuring compliance and providing safe, ethical dental care. Dental burs, as surgical instruments, are subject to stringent standards designed to protect patients from potential harm. These standards encompass everything from manufacturing practices to packaging and labeling, ensuring the bur’s effectiveness and sterility.
Key regulatory bodies, such as the FDA in the United States, play a pivotal role in overseeing dental device safety. They establish guidelines for product development, testing, and distribution, guaranteeing that dental burs meet specific quality and performance criteria. Staying informed about these regulations is crucial for dental practices to maintain adherence to legal requirements, uphold patient safety, and avoid any legal complications.
Compliance: Essential Steps for Dentists
Graft, dat blanda,, drabnie, dat bελ.
#—|
using.
N.,..
Siap.
—モجو, o dירות, blehnie.
“`
Global Standards: A Comparative Study

In the global medical device landscape, regulatory requirements vary across regions, but one constant is the need for safety and quality assurance. When it comes to dental burs, a crucial component in dental procedures worldwide, understanding these variations is essential. Dental burs are subject to stringent regulations, ensuring their efficacy and biocompatibility. Global standards, such as ISO (International Organization for Standardization) guidelines, provide a framework for manufacturers to adhere to, promoting consistency in product quality.
A comparative study of regulatory requirements reveals that while there might be differing nuances, the core principles remain similar. For instance, both the US Food and Drug Administration (FDA) and the European Union’s Medical Devices Regulation (MDR) require rigorous clinical evaluation and adherence to essential safety and performance criteria. This ensures that dental burs, regardless of their origin, meet stringent standards, offering dentists and patients worldwide a reliable and safe solution for various dental procedures.
Safety Protocols: Protecting Patients and Practitioners
In the realm of dental healthcare, safety protocols are paramount to protect both patients and practitioners. One critical aspect is the regulation and proper use of dental burs—small, rotating instruments used for various procedures. These tools must meet stringent standards to ensure they are safe, effective, and free from defects that could lead to patient harm.
Regulatory bodies mandate specific safety measures, including rigorous testing, quality control processes, and clear instructions for their usage. Dental practitioners are responsible for adhering to these guidelines, ensuring regular maintenance, and staying informed about any recalls or updates related to dental burs. By prioritizing safety protocols, the dental community contributes to a more secure environment for patients and professionals alike.
Ongoing Training: Keeping Up with Regulations

Staying current with regulatory requirements is non-negotiable in the healthcare industry, particularly for dental practices. Ongoing training plays a pivotal role in ensuring that dental professionals and staff are well-versed in the latest regulations pertaining to dental burs and other equipment. This continuous learning approach helps to prevent costly mistakes and potential legal issues.
Dental practices should implement regular workshops or webinars focused on regulatory updates. Such sessions can cover new guidelines for waste disposal, infection control protocols, and safety standards related to dental burs. Engaging in ongoing training not only keeps the team informed but also fosters a culture of compliance within the practice.
Regulatory requirements for dental burs are vital to ensuring patient safety and quality care. By understanding the legal framework, implementing stringent compliance measures, adhering to global standards, and prioritizing safety protocols, dentists can navigate the complex landscape of regulations effectively. Ongoing training serves as a cornerstone in this process, enabling professionals to stay abreast of evolving guidelines and maintain the highest standards in their practice. Embracing these steps ensures that dental burs are not just compliant but also contribute to the overall advancement of dental healthcare.